

Z eff Zshielding (blocking positive charge by other electrons). For most atoms, the inner electrons partially shield/block the outer electrons from the pull of the nucleus, and thus. The reason is the same as for atomic radii: shielding by filled inner shells produces little change in the effective nuclear charge felt by the outermost electrons. Effective nuclear charge, Z eff is the pull exerted on a specific electron by the nucleus, taking into account any electronelectron repulsions. Ionic radii follow the same vertical trend as atomic radii that is, for ions with the same charge, the ionic radius increases going down a column. Shannon, “Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides,” Acta Crystallographica 32, no. This is why all anions (negative ions) are larger than they were as neutral atoms.\): Ionic Radii (in Picometers) of the Most Common Ionic States of the s-, p-, and d-Block Elements.Gray circles indicate the sizes of the ions shown colored circles indicate the sizes of the neutral atoms. This causes the electron cloud to spread out, because each electron experiences a lower effective nuclear charge.
When electrons are added to an atom without a change in the number of protons, the electrons experience additional repulsion among them. This would give the ion an electron configuration like the noble gas at the end of their row on the periodic table. Given the fact that both elements are in the same period, what information from the PES. Nitrogen atoms have a smaller nuclear charge and coulombic attraction than oxygen atoms. Most nonmetals gain enough electrons, if they can, to fill their valence energy level. The effective nuclear charge of an atom is primarily affected by (A) inner core electrons (B) outer electrons (C) nuclear charge only. Nonmetals naturally form anions, negative ions, by gaining one or more electrons. This is why all cations (positive ions) are smaller than they were as neutral atoms. As you may expect, when the outermost energy level of electrons is emptied, the remaining electron cloud is smaller.

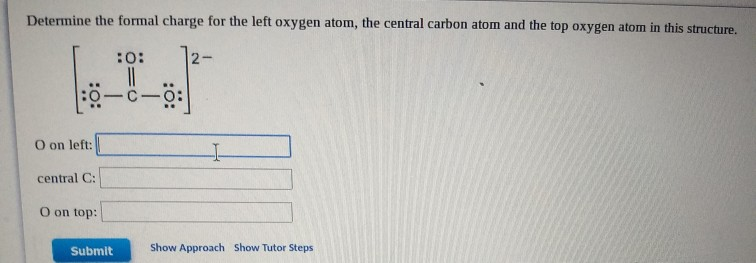
Most metals lose enough electrons to empty out their valence, giving the ion an electron configuration of the noble gas from the previous period. Effective nuclear charge, Z Z where, Z atomic number, shielding or shielding constant. Metals naturally form cations, positive ions, by losing one or more electrons. Effective Nuclear Charge Of Oxygen - The effective nuclear charge can be defined as the actual nuclear charge (Z) minus the screening effect caused by electrons intervening between the nucleus and the valence electrons. A positive ion is called a cation, and a negative ion is called an anion. Determine the core charge for an oxygen atom and use it. An ionic radius is one-half the diameter of an ion. The core charge an atom is equal to the net charge of the nucleus and the inner (nonvalence) electrons. The periodic table below shows a comparison of the radii of the most commonly formed ion for each element. The equation 9 - 2 = 7 gives the estimated effective nuclear charge felt by each of fluorine's seven valence electrons. This is because the total nuclear charge (+9) is shielded by the two core electrons in the first energy level. What is the effective nuclear charge felt by the valence electrons in fluorine? Answer: The valence electrons feel an effective nuclear charge of about +7. Now take a look at the fluorine (F) atom, which has a total of nine protons and nine electrons. This means that each of the valence electrons in oxygen feels an effective nuclear charge of about +6. The +8 charge of oxygen's nucleus is shielded by the two core electrons, and it is also decreased slightly by the repulsion between the electrons themselves. The electrons that are in oxygen's valence energy level would be shielded from the nucleus by the two core electrons in the first energy level. The repulsion between the two negative electrons does slightly decrease their effective nuclear charge, but it can still be rounded to +8.
#Effective nuclear charge of oxygen full
The two electrons in the first energy level feel almost a full +8 charge because there is nothing between these electrons and the nucleus. The nucleus itself has a +8 charge because it has eight protons, and anything in its vicinity will feel that charge.

Let's take a look at the electrons in oxygen for an example of effective nuclear charge.


 0 kommentar(er)
0 kommentar(er)
